
Excessive Sweating
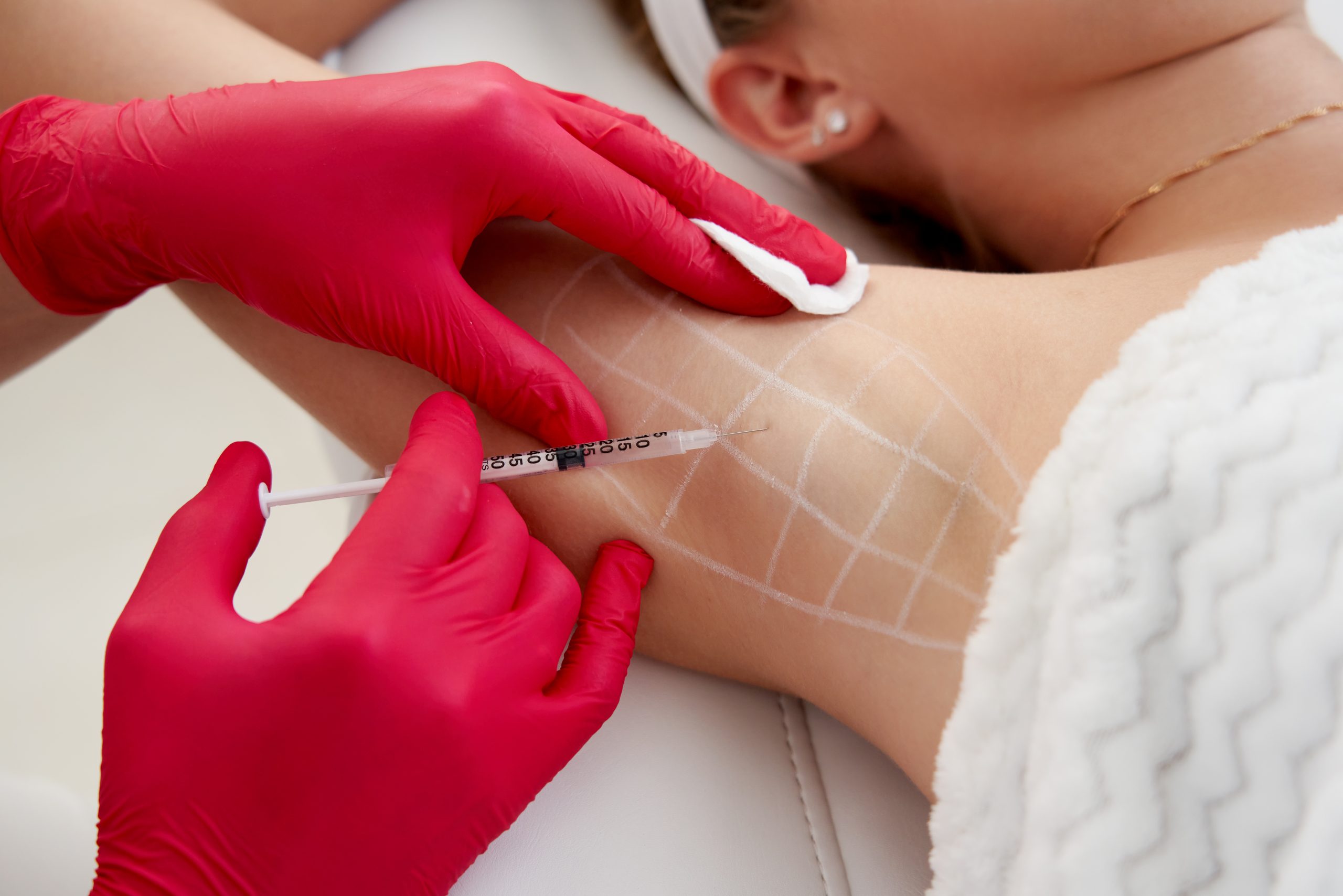
It temporarily blocks the function of sweat glands by blocking the secretion of the neurotransmitter acetylcholine in areas where sweat is excessively secreted. Botulinum toxin is injected into sweaty areas such as underarm to reduce sweat secretion and relieve discomfort.
Questions & Answers
When can I see the effect?
You can see that the amount of sweat has decreased after 1-2 weeks.
How long the effect lasts?
One treatment lasts about 6 months on average, so 1-2 treatments a year are recommended.
Are there anything I need to be avoid after the treatment?
- Avoid deodorant or rubbing the area for 24 hours
- Wear loose-fitting clothes to prevent any rubbing of the area
- Avoid IPL & waxing for 2 weeks
Dysport®: Clostridium botulinum type A toxin-haemagglutinin complex (300 or 500 IPSEN UNITS/vial). Indications: Focal spasticity affecting the upper and lower limbs in adults; spasmodic torticollis in adults; lower limb focal spasticity in children two years of age or older; blepharospasm in adults; hemifacial spasm in adults; moderate to severe glabellar lines and/or lateral canthal lines (crow’s feet) in adults; symptomatic treatment of axillary hyperhidrosis. Contraindications: Hypersensitivity to any component; pregnancy; diagnosis of myasthenia gravis or Eaton-Lambert (myasthenic) syndrome; infection at proposed injection site. Precautions: The units of Dysport are not interchangeable with other preparations of botulinum type A toxin. Use lowest effective dose and do not exceed recommended dosages and frequencies of administration; adverse effects resulting from the distribution of toxin to sites remote from the site of administration have been reported (excessive muscle weakness); use with caution in patients with: evidence of any defect in neuro-muscular transmission, increased risk of falls (eg. elderly), breathing and swallowing difficulties, and prolonged bleeding times; rare aspiration risk with patients who have a chronic respiratory disorder; rare occurrence of antibody formation to botulinum toxin; contains small amount of human albumin so the risk of transmission of viral infection cannot be excluded; use with caution during pregnancy, not recommended in lactation; apply extreme caution when treating children with significant neurologic debility, dysphagia, or recent history of aspiration pneumonia or lung disease. Adverse Effects: common to very common depending on indication: asthenia, fatigue, influenza-like illness, injection site pain/bruising/swelling/haematoma/erythema/pruritis/rash, muscular weakness, muscular weakness of adjacent muscle to the area of injection, fall, headache, dizziness, facial paresis, vision blurred, visual acuity reduced, dysphagia, dysphonia, dyspnoea, dry mouth, neck pain, musculoskeletal pain or stiffness, myalgia, pain in extremity, urinary incontinence, gait disturbance, ptosis, diplopia, dry eyes, lacrimation increased, eyelid oedema, periorbital haematoma, asthenopia, muscle twitching, compensatory sweating. Dose: The units of Dysport are not interchangeable with other preparations of botulinum type A toxin. Recommended minimum interval between treatments is 12 weeks. Focal spasticity of upper limb: 1000 units (up to 1500 units when shoulder muscles also injected). Focal spasticity of lower limb: up to 1500 units. Spasmodic torticollis: 250-1000 units. Focal spasticity of lower limb in children aged 2 years of age and older: 15-30 units/kg bodyweight, up to a maximum of 1000 units. Blepharospasm & hemifacial spasm: 40-120 units/eye. Glabellar lines: 50 units. Lateral canthal lines: 30 units/side. Axillary hyperhidrosis: 100-200 units/axillae. Administration: Intramuscular injection for all indications except blepharospasm/hemifacial spasm where it is injected subcutaneously (alternative route for glabellar lines) and axillary hyperhidrosis where it is administered intradermally. Storage: 2C – 8C. (Refrigerate. Do not freeze.) The product does not contain an anti-microbial agent. Reconstituted product to be used as soon as possible (within 24 hrs when stored at 2C – 8C). Galderma Australia Pty Ltd, Suite 4, 13B Narabang Way, Belrose NSW 2085. ABN 12 003 976 930. Distributed in NZ by Healthcare Logistics, 58 Richard Pearse Drive, Airport Oaks, Mangere 2022, NZ. Phone: 1800 144 944 (AUS) 0800 174 104 (NZ) Fax: +61 (2) 9986 1699. Sponsor: Ipsen Pty Ltd, Level 2, Building 4, Brandon Office Park, 540 Springvale Road, Glen Waverley, Victoria 3150 Australia. Distributed by Galderma Australia Pty Ltd for the glabellar lines and lateral canthal lines indications only. Dysport® is a trademark of Ipsen Pty Ltd. Galderma trademark is owned by Galderma Holding S.A.





